Drugs in Development
Speeding the Pace of Progress to
Deliver Treatments and Cures
The PMSF actively builds relationships and works closely with drug developers to encourage those with promising technologies to add Phelan-McDermid syndrome drugs to their pipeline. These are drugs which are currently being tested and are not yet in clinical trials. For current trials, see our Clinical Trials page.
Drug development in Phelan-McDermid syndrome generally targets one of four categories:
Therapeutic Strategies
Designed to address errors in the SHANK3 and/or other 22q13 genes
Examples: Gene Therapy, RNA therapy (ASOs)
Designed to improve the function of neurons or networks in the brain.
Examples: Growth Hormone, IGF-1, NNZ-2591
Designed to address individual symptoms (GI disorders, epilepsy, sleep)
Examples: Soticlestat (epilepsy), AMO-01 (epilepsy)
Multiple approaches combined together
After a drug is developed, it is tested in laboratory models for safety and if it makes a positive impact on outcomes related to Phelan-McDermid syndrome. Model systems can be cells or animals that imitate the syndrome. This step is called pre-clinical research. If this phase goes well, the group making or sponsoring the drug can submit data and ask the FDA or another regulatory agency for permission to start clinical trials. The pre-clinical stage usually takes a long time, often years. You can click on each drug being tested below to get more information.
Phelan-McDermid Syndrome Drugs in Development
Jaguar Gene Therapy Targets SHANK3 in the Development of JAG201
| Drug | Phase | SPONSOR | Target |
|---|---|---|---|
| JAG201 | Clinical Trials | Jaguar Gene Therapy | Gene Therapy |
Neuren Pharmaceuticals Advances NNZ-2591 Targeting Brain Connectivity in Phelan-McDermid Syndrome
| Drug | Phase | SPONSOR | Target |
|---|---|---|---|
| NNZ-2591 | Clinical Trials | Neuren Pharmaceuticals | Brain Cell/Circuit Connectivity |
PYC Therapeutics has added an ASO (PYC-002) for Phelan-McDermid syndrome to their pipeline
| Drug | Phase | SPONSOR | Target |
|---|---|---|---|
| PYC-002 | Pre-Clinical Research | PYC Theraputics | RNA Therapy |
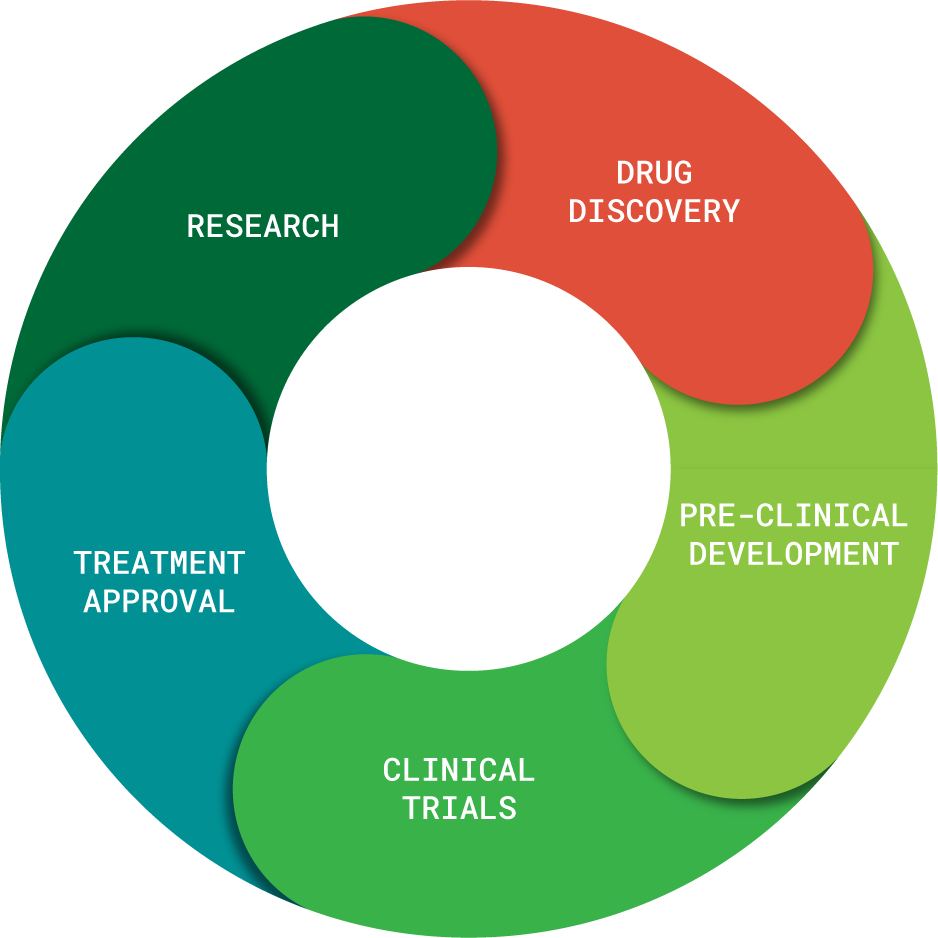
Phase of Drug Development
We envision drug development as a cycle rather than as stairs. New discoveries are constantly being made which can inform new treatments. For example, a new drug for Phelan-McDermid syndrome discovered today could be as important in the future as a drug in clinical trials today. The PMSF supports every stage of research equally.
We say treatments and cures in plural because no two people have the same exact symptoms. Individuals with Phelan-McDermid syndrome may need different treatments, and the same person might need multiple types of treatments.
Educational Materials
Playlist

5:38

1:10:00

1:10:00
Industry Partners
Interested in working with PMSF while developing a drug for Phelan-McDermid syndrome? Check out our Research Partners page for available resources, and contact our Chief Science Officer.



